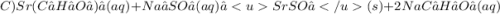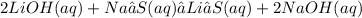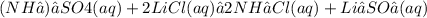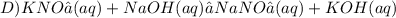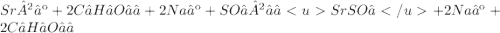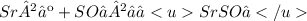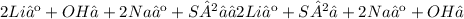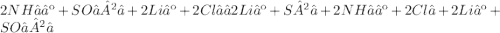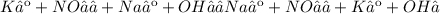Give two examples (each) of strong electrolyte, weak electrolyte, and nonelectrolyte. 2. predict the products for reactions below. which of the following reaction(s) produce a precipitate? a) lioh + na2s b) (nh4)2so4 + licl c) sr(c2h3o2)2 + na2so4 d) kno3 + naoh e) none of the above solution pairs will produce a precipitate. 3. what are the spectator ions in the precipitation reaction you chose above? 4. write the molecular, complete ionic, and net ionic equations for the reactions in q2.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 02:30
List four observations that indicate that a chemical reaction may be taking place
Answers: 1

Chemistry, 22.06.2019 05:50
What are transitions between a liquid and gas called? identify which way they are transitioning
Answers: 2

Chemistry, 22.06.2019 17:00
How can a give a full method for the experiment of separating sand from water by filtration? 1-materials 2-steps 3-conclusion also for water and salt separated by the evaporation or distillation process
Answers: 1

Chemistry, 23.06.2019 02:20
Which of the following will cause an increase in the acceleration of an object? increase force decrease force increase mass decrease mass
Answers: 1
You know the right answer?
Give two examples (each) of strong electrolyte, weak electrolyte, and nonelectrolyte. 2. predict the...
Questions

History, 21.11.2019 05:31




Mathematics, 21.11.2019 05:31





Biology, 21.11.2019 05:31


English, 21.11.2019 05:31




Mathematics, 21.11.2019 05:31



Mathematics, 21.11.2019 05:31

English, 21.11.2019 05:31