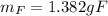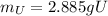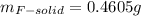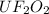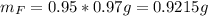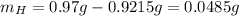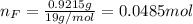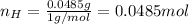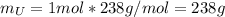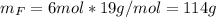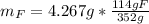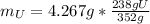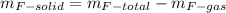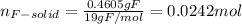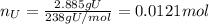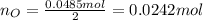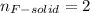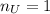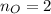Water is added to 4.267 g uf6. the only products are 3.730 g of a solid containing only uranium, oxygen and fluorine and 0.970 grams of gas. the gas is 95.0% by mass fluorine and the remainder is hydrogen.
(a) from the data, determine the molecular formula of the gas (same as the empirical formula)
(b) what is the mass of fluorine in uf6?
(c) what is the mass of uranium in uf6?
(d) what is the mass of fluorine in the solid product?
(e) determine the molecular formula (same as empirical) for the solid product the write a balanced chemical equation for the reaction.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 14:50
Complete the following statements to describe solids, liquids, and gases. select the correct answer from each drop-down menu. a solid a definite volume and a definite shape. a liquid a definite volume and a definite shape. a gas a definite volume and a definite shape
Answers: 1

Chemistry, 22.06.2019 20:00
I’m an electrically neutral atomic any element, there are equal numbers of
Answers: 2

Chemistry, 23.06.2019 04:31
How does a sample of helium at 15 degree celsius compare to a sample of helium at 215 k? a) the helium at 15 degrees celsius has a higher average kinetic energy that the sample at 215 k. b) the helium at 15 degrees celsius has lower nuclear energy that the sample at 215 k. c) the helium at 15 degrees celsius has slower- moving atoms that the sample at 215 k. d) the helium at 15 degrees celsius has smaller atoms than the sample at 215 k.
Answers: 1

You know the right answer?
Water is added to 4.267 g uf6. the only products are 3.730 g of a solid containing only uranium, oxy...
Questions


Mathematics, 31.12.2020 03:30

Physics, 31.12.2020 03:30

Mathematics, 31.12.2020 03:30

English, 31.12.2020 03:30

Biology, 31.12.2020 03:30

Mathematics, 31.12.2020 03:30



English, 31.12.2020 03:30



English, 31.12.2020 03:30

English, 31.12.2020 03:30


Chemistry, 31.12.2020 03:30




Mathematics, 31.12.2020 03:30