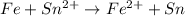Chemistry, 26.11.2019 05:31 ariannecama
Agalvanic cell with e o cell = 0.30 v can be constructed using an iron electrode in a 1.0 m fe(no3)2 solution, and either a tin electrode in a 1.0 m sn(no3)2 solution, or a chromium electrode in a 1.0 m cr(no3)3 solution even though sn2+/sn and cr3+/cr have different reduction potentials. give the overall balanced reaction for fe-sn cell. do not include the states of matter.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 17:30
Which uses electromagnetic radiation to discover the properties and composition of bodies in space? space probe space station space shuttle space observatory
Answers: 2

Chemistry, 21.06.2019 21:00
Which of the following compounds does not contain molecules? question 2 options: co2 h2 nacl h2o
Answers: 1

Chemistry, 22.06.2019 12:30
According to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? view available hint(s) according to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? trigonal bipyramidal tetrahedral square planar determination of electron geometry requires information on whether the electron groups are lone pairs or bonding groups.
Answers: 2

Chemistry, 22.06.2019 14:00
650.j is the same amount of energy as? 2720cal1550cal650.cal2.72cal
Answers: 2
You know the right answer?
Agalvanic cell with e o cell = 0.30 v can be constructed using an iron electrode in a 1.0 m fe(no3)2...
Questions

Mathematics, 12.03.2021 19:10



History, 12.03.2021 19:10



Mathematics, 12.03.2021 19:10


Mathematics, 12.03.2021 19:10




Mathematics, 12.03.2021 19:20

Biology, 12.03.2021 19:20

Mathematics, 12.03.2021 19:20

Mathematics, 12.03.2021 19:20

Mathematics, 12.03.2021 19:20

English, 12.03.2021 19:20

English, 12.03.2021 19:20

Mathematics, 12.03.2021 19:20

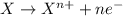
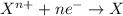
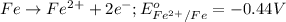
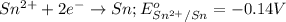
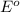 potential will always get reduced and will undergo reduction reaction. Here, zinc will always undergo reduction reaction will get reduced.
potential will always get reduced and will undergo reduction reaction. Here, zinc will always undergo reduction reaction will get reduced.