
Chemistry, 26.11.2019 05:31 jocelynmarquillo1
vinegar is a solution ofacetic acid, ch3cooh, dissolved in water. a 5.54-gsample of vinegar was neutralized by 30.10 ml of 0.100 m naoh. whatis the percent by weight of acetic acid in the vinegar?
why is the answer3.26%?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 07:30
Label a-f based on the table using c for concentrated and d for dilute
Answers: 2

Chemistry, 22.06.2019 09:00
Suppose you have designed a new thermometer called the x thermometer. on the x scale the boiling point of water is 129 ? x and the freezing point of water is 13 ? x. part a at what temperature are the readings on the fahrenheit and x thermometers the same?
Answers: 1

Chemistry, 23.06.2019 00:00
How do you determine the percent yield of a chemical reaction
Answers: 1

Chemistry, 23.06.2019 00:50
50 points! need answer asap. what type of organic compound contains the following functional group? (2 points)
Answers: 3
You know the right answer?
vinegar is a solution ofacetic acid, ch3cooh, dissolved in water. a 5.54-gsample of vinegar was neut...
Questions

English, 23.08.2019 04:30

History, 23.08.2019 04:30

Health, 23.08.2019 04:30


Mathematics, 23.08.2019 04:30


English, 23.08.2019 04:30

English, 23.08.2019 04:30


Computers and Technology, 23.08.2019 04:30

Biology, 23.08.2019 04:30


Mathematics, 23.08.2019 04:30






History, 23.08.2019 04:30


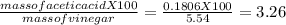 %
%


