
Chemistry, 26.11.2019 06:31 Marcus2935
Here is the information: a 1.2516 gram sample of a mixture of caco3 and na2so4 was analyzed by dissolving the sample and adding c2o42- to completely precipitate the ca2+ as cac2o4. the cac2o4 was dissolved in sulfuric acid and the resulting h2c204 was titrated with a standard kmno4 solution.
this is the balanced equation: 3h2c2o4 + 2h+ + mno4- > mn2+ + 4h20 + 6co2
another part: the titration of the h2c2h4 obtained required 35.62 milliliters of .1092 molar mno4- solution. calculate the number of moles of h2c2o4 that reacted with the mno4-. i think the correct answer is .01167 mol h2c2o4. this is not my question.
next part: calculate the number of moles of caco3 in the original sample. based on the answer to the part above, the answer is .003883 mol caco3 (i think this is correct, however this is not my question)
calculate the percentage by weight of caco3 in the original sample.
find this based off of the .01167 mol h2c2o4 and the .003883 mol caco3. basically just use the answers above to solve for this.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 13:10
Which equation represents a fission reaction? o "9n+h—150 o 235u + n—190cs + rb+25 o be + he—1c + in o 28 np —> 2390 pute
Answers: 1


Chemistry, 22.06.2019 12:00
Which statement best explains the relationship between an area is geography and the temperature of its surface water
Answers: 1

You know the right answer?
Here is the information: a 1.2516 gram sample of a mixture of caco3 and na2so4 was analyzed by diss...
Questions

Mathematics, 01.10.2019 03:40


History, 01.10.2019 03:40



Spanish, 01.10.2019 03:40


Biology, 01.10.2019 03:40

Biology, 01.10.2019 03:40

History, 01.10.2019 03:40

History, 01.10.2019 03:40

English, 01.10.2019 03:40

Biology, 01.10.2019 03:40

Mathematics, 01.10.2019 03:40




Physics, 01.10.2019 03:40


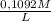 =
= 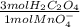 = 0,01167 mol of H₂C₂O₄
= 0,01167 mol of H₂C₂O₄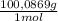 = 1,168 g of CaCO₃
= 1,168 g of CaCO₃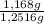 ×100 = 93,32 % (w/w)
×100 = 93,32 % (w/w)

