Chemistry, 26.11.2019 06:31 maddiehope6893
The mole fraction of co2 in a certain solution with h2o as the solvent is 3.6 × 10−4. what is the approximate molality of co2 in this solution? a.0.00036 m. b.0.0065 m. c.0.020 m. d.2.0 × 10−5 m. e.6.5 m

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 18:30
Calculate the change in entropy if br2(l) is converted to br2(g). s° for br2(l) = 152.2 j/(mol•k) s° for br2(g) = 245.5 j/(mol•k) s° for br(g) = 175.0 j/(mol•k)
Answers: 3

Chemistry, 21.06.2019 18:30
Which orbitals form a pi bond? a.the s orbital and three p orbitals b.the s orbital and two p orbitals c.overlapping p orbitals d.overlapping hybrid orbitals
Answers: 2

Chemistry, 22.06.2019 06:30
Identify the missing numbers below to show the result of multiplying the numbers (1.6 × 10-19)(5.0 × 106) = c × 10d
Answers: 1

Chemistry, 22.06.2019 12:30
Which element has the lowest electronegativity? calcium(ca) gallium(ga) selenium(se) bromine(br)
Answers: 1
You know the right answer?
The mole fraction of co2 in a certain solution with h2o as the solvent is 3.6 × 10−4. what is the ap...
Questions





Mathematics, 29.01.2022 20:20


Mathematics, 29.01.2022 20:20


Physics, 29.01.2022 20:20

Mathematics, 29.01.2022 20:20

Physics, 29.01.2022 20:30

Mathematics, 29.01.2022 20:30


Chemistry, 29.01.2022 20:30


English, 29.01.2022 20:30


French, 29.01.2022 20:30


Mathematics, 29.01.2022 20:30

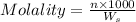
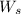 = weight of solvent in g
= weight of solvent in g 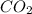 is =
is = 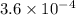 i.e.
i.e.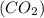 =
=