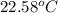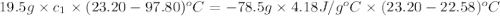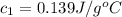Chemistry, 26.11.2019 06:31 JaleahOwens13
In the laboratory a student uses a "coffee cup" calorimeter to determine the specific heat of a metal.
she heats 19.5 grams of tungsten to 97.80°c and then drops it into a cup containing 78.3 grams of water at 22.58°c. she measures the final temperature to be 23.20°c.
assuming that all of the heat is transferred to the water, she calculates the specific heat of tungsten to be j/g°c.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 17:30
What are the major products produced in the combustion of c10h22 under the following conditions? write balanced chemical equations for each. a. an excess of oxygen b. a slightly limited oxygen supply c. a very limited supply of oxygen d. the compound is burned in air
Answers: 2

Chemistry, 23.06.2019 01:00
Atoms contain subatomic particles called protons and neutrons. when these protons and neutrons spilt, a lot of energy is released
Answers: 3

Chemistry, 23.06.2019 07:00
What are the trends and exceptions to the trends in electron affinity?
Answers: 1

Chemistry, 23.06.2019 07:30
Type the letter that represents the correct location for each particle type below. the neutron is found at __ the electron is found at __ the proton is found at __
Answers: 2
You know the right answer?
In the laboratory a student uses a "coffee cup" calorimeter to determine the specific heat of a meta...
Questions

Mathematics, 27.01.2020 21:31



Mathematics, 27.01.2020 21:31


Mathematics, 27.01.2020 21:31

Mathematics, 27.01.2020 21:31



History, 27.01.2020 21:31

History, 27.01.2020 21:31




Social Studies, 27.01.2020 21:31



Geography, 27.01.2020 21:31

Social Studies, 27.01.2020 21:31

Biology, 27.01.2020 21:31

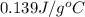
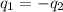
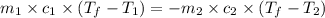
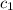 = specific heat of tungsten = ?
= specific heat of tungsten = ?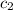 = specific heat of water =
= specific heat of water = 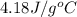
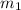 = mass of tungsten = 19.5 g
= mass of tungsten = 19.5 g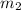 = mass of water = 78.5 g
= mass of water = 78.5 g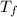 = final temperature =
= final temperature = 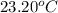
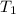 = initial temperature of tungsten =
= initial temperature of tungsten = 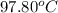
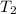 = initial temperature of water =
= initial temperature of water =