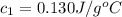Chemistry, 26.11.2019 06:31 ejhoff4347
A23.9 g sample of iridium is heated to 89.7°c, and then dropped into 20.0 g of water in a foam-cup calorimeter. the temperature of the water went from 20.1°c to 22.6°c. calculate the specific heat of iridium (specific heat of water = 4.18 j/g.°c)

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 06:30
Melting and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1

Chemistry, 22.06.2019 13:50
How does the motion of particles in a gas change as the gas cools
Answers: 2


Chemistry, 22.06.2019 14:20
7. in the cycle, a virus integrates its dna into the host's dna, and its dna is replicated when the host dna is replicated. a. infectious b. retroviral c. lysogenic d.lytic
Answers: 1
You know the right answer?
A23.9 g sample of iridium is heated to 89.7°c, and then dropped into 20.0 g of water in a foam-cup c...
Questions




English, 31.01.2020 14:53


History, 31.01.2020 14:53




Health, 31.01.2020 14:53


Chemistry, 31.01.2020 14:53


Spanish, 31.01.2020 14:53



Mathematics, 31.01.2020 14:53


History, 31.01.2020 14:53

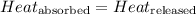
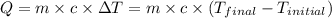
![m_1\times c_1\times (T_{final}-T_1)=-[m_2\times c_2\times (T_{final}-T_2)]](/tpl/images/0391/1986/09236.png) ......(1)
......(1)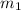 = mass of iridium = 23.9 g
= mass of iridium = 23.9 g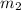 = mass of water = 20.0 g
= mass of water = 20.0 g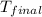 = final temperature = 22.6°C
= final temperature = 22.6°C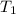 = initial temperature of iridium = 89.7°C
= initial temperature of iridium = 89.7°C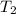 = initial temperature of water = 20.1°C
= initial temperature of water = 20.1°C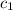 = specific heat of iridium = ?
= specific heat of iridium = ?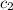 = specific heat of water = 4.18 J/g°C
= specific heat of water = 4.18 J/g°C![23.9\times c_1\times (22.6-89.7)=-[20\times 4.18\times (22.6-20.1)]](/tpl/images/0391/1986/4699c.png)