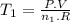Chemistry, 26.11.2019 06:31 kinziemadison12
Achemist prepares a sample of helium gas at a certain pressure, temperature and volume and then removes all but a fourth of the gas molecules (only a fourth remain). how must the temperature be changed (as a multiple of t1) to keep the pressure and the volume the same? a. t2=1/16t1b. t2=2t1c. t2=16t1d. t2= 1/2t1e. t2=4t1f. none of theseg. t2=1/4t1

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 02:20
Compared with the freezing-point depression of a 0.01 m c6h12o6 solution, the freezing-point depression of a 0.01 m nacl solution is
Answers: 1

Chemistry, 22.06.2019 08:30
Sally is making a model of a magnesium atom with an atomic mass number of 24 for her chemistry class. she has foam balls for the protons, neutrons, and electrons. she has added 6 neutrons to her model so far. how many more neutrons does she need to add to complete her neutral atom of magnesium?
Answers: 1

Chemistry, 22.06.2019 10:10
For the reaction, 4 a(g) + 3 b(g) => 2 c(g), the following data were obtained at constant temperature. experiment initial[a],mol/l initial [b],mol/l initial rate,m/min 1 0.200 0.150 5.00 2 0.400 0.150 10.0 3 0.200 0.300 10.0 4 0.400 0.300 20.0 which of the following is the correct rate law for the reaction? 1. rate = k[a]2[b]2 2. rate = k[a][b] 3. rate = k[a]2[b] 4. rate = k[a][b]2
Answers: 3

Chemistry, 22.06.2019 16:00
Inside a flashbulb, oxygen surrounds a thin coil of magnesium. when the flashbulb is set off, a chemical reaction takes place in which magnesium combines with oxygen to form magnesium oxide. which of the chemical equations matches the reaction above? a. mg + o2 mgo2 + energy b. 2mg + o mg2o + energy c. 2mg + o2 2mgo + energy d. mg + o mgo + energy
Answers: 1
You know the right answer?
Achemist prepares a sample of helium gas at a certain pressure, temperature and volume and then remo...
Questions






History, 26.10.2019 06:43

Mathematics, 26.10.2019 06:43


Social Studies, 26.10.2019 06:43


Social Studies, 26.10.2019 06:43


Mathematics, 26.10.2019 06:43

Mathematics, 26.10.2019 06:43

English, 26.10.2019 06:43





English, 26.10.2019 06:43