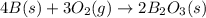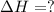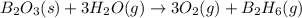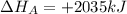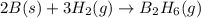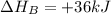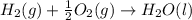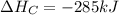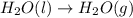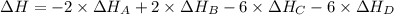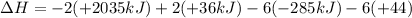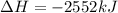Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 18:00
Sylvanite is a mineral that contains 28.0% gold by mass. how much sylvanite would you need to dig up to obtain 77.0 g of gold? explain how you got your answer and the steps you took. you
Answers: 3

Chemistry, 21.06.2019 19:00
Asyringe contains 56.05 ml of gas at 315.1 k. what volume will that gas occupy if the temperature is increased to 380.5 k? a) 12.41 b) 46.42 c) 67.68 d) 81.74
Answers: 1


Chemistry, 22.06.2019 10:30
Geothermal energy for industrial use is available almost anywhere. a.true b.false
Answers: 2
You know the right answer?
Calculate the enthalpy of the reaction
4b(s)+3o2(g)→2b2o3(s)
given the following pertine...
4b(s)+3o2(g)→2b2o3(s)
given the following pertine...
Questions

Mathematics, 14.12.2020 21:10




Mathematics, 14.12.2020 21:10

Arts, 14.12.2020 21:10



Engineering, 14.12.2020 21:10





English, 14.12.2020 21:10



Social Studies, 14.12.2020 21:10

Computers and Technology, 14.12.2020 21:10

English, 14.12.2020 21:10