
Chemistry, 26.11.2019 07:31 emanuelmorales1515
The ksp for zn(oh)2 is 5.0 x 10-17. determine the molar solubility of zn(oh)2 in a buffer solution with a ph of 11.5.
a) 5.0 x 106
b) 1.2 x 10-12
c) 1.6 x 10-14
d) 5.0 x 10-12
e) 5.0 x 10-17

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 08:30
For each of the compounds below, show that the charges on the ions add up to zero. a. kbr b. cao c. li(2)o d. cacl(2) e. alcl(3)
Answers: 2

Chemistry, 22.06.2019 12:00
In the following redox reaction which is the oxidizing agent and which is the reducing agent? alcl3 + na nacl + al oxidizing agent = reducing agent =
Answers: 1

Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium
Answers: 1

Chemistry, 22.06.2019 20:00
Suppose that some of the compound spilled out of the crucible after it was heated. would that cause the percent by mass of water in the compound determined by the experiment to be too low, too high, or unchanged? briefly explain your answer.
Answers: 1
You know the right answer?
The ksp for zn(oh)2 is 5.0 x 10-17. determine the molar solubility of zn(oh)2 in a buffer solution w...
Questions

English, 02.11.2019 02:31

Mathematics, 02.11.2019 02:31






Mathematics, 02.11.2019 02:31


Geography, 02.11.2019 02:31

Social Studies, 02.11.2019 02:31



Mathematics, 02.11.2019 02:31



Mathematics, 02.11.2019 02:31



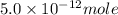
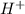 concentration.
concentration.![pH=-\log [H^+]](/tpl/images/0391/2894/37e81.png)
![11.5=-\log [H^+]](/tpl/images/0391/2894/f3755.png)
![[H^+]=3.16\times 10^{-12}M](/tpl/images/0391/2894/3012d.png)
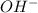 concentration.
concentration.![[H^+][OH^-]=K_w](/tpl/images/0391/2894/55f9c.png)
![3.16\times 10^{-12}\times [OH^-]=1.0\times 10^{-14}](/tpl/images/0391/2894/64b28.png)
![[OH^-]=3.16\times 10^{-3}M](/tpl/images/0391/2894/38f53.png)
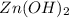 .
.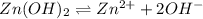
![K_{sp}=[Zn^{2+}][OH^-]^2](/tpl/images/0391/2894/b302a.png)
![5.0\times 10^{-17}=[Zn^{2+}]\times (3.16\times 10^{-3})^2](/tpl/images/0391/2894/0b771.png)
![[Zn^{2+}]=5.0\times 10^{-12}M](/tpl/images/0391/2894/e914d.png)



