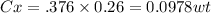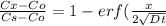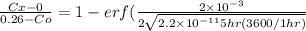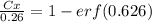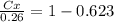Chemistry, 26.11.2019 07:31 ravenl1001
Nitrogen from a gaseous phase is to be diffused into pure iron at 700°c. if the surface concentration is maintained at 0.26 wt% n, what will be the concentration (in weight percent) 2.8 mm from the surface after 6.3 h? the diffusion coefficient for nitrogen in iron at 700°c is 2.2 × 10-10 m2/s.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 03:00
Which best describes how johannes kepler developed his laws of planetary motion
Answers: 3

Chemistry, 22.06.2019 06:00
There are 6.022, 104 atoms of hg in 1 mole of hg the number of atoms in 45 moles of hg can be found by multiplying 4.5 by 6.022, 102 which is the number of atoms in 4.5 moles of hg, correctly written in scientific notation with the correct number of significant figures? 0 21,109 0 21,100 271, 1024 27.099, 100 mark this and retum save and exit submit
Answers: 1

Chemistry, 22.06.2019 08:30
Which change in temperature is the smallest? a change of 1 thomson degree a change of 1 kelvin degree a change of 1 fahrenheit degree a change of 1 celsius degree
Answers: 1

Chemistry, 22.06.2019 17:30
The polymer used for the nonstick surface of cooking utensils is 24.0%c and 76%f by mass. what is the empirical formula of this polymer?
Answers: 2
You know the right answer?
Nitrogen from a gaseous phase is to be diffused into pure iron at 700°c. if the surface concentratio...
Questions

English, 21.05.2020 19:58


Social Studies, 21.05.2020 19:58

Mathematics, 21.05.2020 19:58

History, 21.05.2020 19:58







Mathematics, 21.05.2020 19:58






Mathematics, 21.05.2020 19:58

Biology, 21.05.2020 19:58