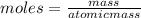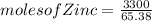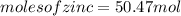Chemistry, 26.11.2019 07:31 loredohome
Galvanized nails are iron nails that have been plated with zinc to prevent rusting. the relevant reaction is
zn2+(aq)+2e−→zn(s)
for a large batch of nails, a manufacturer needs to plate a total zinc mass of 3.30 kg on the surface to get adequate coverage.
part a
how many moles of zinc are in 3.30 kg of zinc?
express your answer to three significant figures and include the appropriate units.
50.5 mol
submithintsmy answersgive upreview part
correct
significant figures feedback: your answer 50.47mol was either rounded differently or used a different number of significant figures than required for this part.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 13:20
Why is an elements atomic mass not listed as a whole number on the periodic table
Answers: 2

Chemistry, 21.06.2019 18:00
What does earth’s rotation on its axis cause? the tides night and day passing of years phases of the moon
Answers: 1

Chemistry, 22.06.2019 01:30
What is the value of keq for the reaction expressed in scientific notation
Answers: 1

Chemistry, 22.06.2019 08:00
Which of the following observations indicates that there is a small, dense, positively charged part in the center of an atom? some uncharged particles are scattered by a gold foil. all uncharged particles are attracted towards a gold foil. all positively charged particles pass straight through a gold foil. some positively charged particles bounce back from a gold foil.
Answers: 2
You know the right answer?
Galvanized nails are iron nails that have been plated with zinc to prevent rusting. the relevant rea...
Questions


Computers and Technology, 29.03.2021 17:30


English, 29.03.2021 17:30

Social Studies, 29.03.2021 17:30


Biology, 29.03.2021 17:30

Mathematics, 29.03.2021 17:30


Computers and Technology, 29.03.2021 17:30



Mathematics, 29.03.2021 17:30


Mathematics, 29.03.2021 17:30


English, 29.03.2021 17:30



Spanish, 29.03.2021 17:30