Chemistry, 26.11.2019 17:31 lanettejohnson355
Using the following data, determine the standard cell potential e^o cell for the electrochemical cell constructed using the following reaction, where zinc is the anode and lead is the cathode.
zn(s) + pb2+(aq) -> zn2+(aq) + pb(s)
half-reaction: standard reduction potential:
zn2+(aq) + 2e- -> zn(s)= -0.763
pb2+(aq) + 2e- -> pb(s)= -0.126
a. -0.889 v
b. +0.889 v
c. +0.637 v
d. +1.274 v
e. -0.637 v

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 23:00
Select the correct answer. given: 2libr + ba → babr2 + 2li in this chemical reaction, 325 grams of barium (ba) react completely. how many moles of lithium (li) are produced? a. 1.18 mol b. 2.37 mol c. 4.73 mol d. 16.4 mol e. 32.9 mol
Answers: 2

Chemistry, 22.06.2019 07:20
The diagrams show objects’ gravitational pull toward each other. which statement describes the relationship between diagram x and y? gravity attracts only larger objects toward one another. gravity attracts larger objects only if they are close to one another. if the masses of the objects increase, then the force between them also increases. if distance between the objects increases, then the amount of force also increases.
Answers: 1

Chemistry, 22.06.2019 19:30
Helium decays to form lithium. which equation correctly describes this decay?
Answers: 2

Chemistry, 22.06.2019 20:30
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 2
You know the right answer?
Using the following data, determine the standard cell potential e^o cell for the electrochemical cel...
Questions

Mathematics, 07.11.2020 14:00

Mathematics, 07.11.2020 14:00


English, 07.11.2020 14:00


Arts, 07.11.2020 14:00


Mathematics, 07.11.2020 14:00

Arts, 07.11.2020 14:00


Mathematics, 07.11.2020 14:00

Social Studies, 07.11.2020 14:00

Mathematics, 07.11.2020 14:00


Mathematics, 07.11.2020 14:00

English, 07.11.2020 14:00

English, 07.11.2020 14:00

Social Studies, 07.11.2020 14:00


Arts, 07.11.2020 14:00

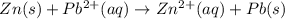
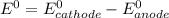
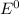 are standard reduction potentials.
are standard reduction potentials.
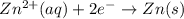 = -0.763
= -0.763
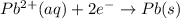 = -0.126
= -0.126![E^0_{[Zn^{2+}/Zn]}=-0.763V](/tpl/images/0391/6528/6b929.png)
![E^0_{[Pb^{2+}/Pb]}=-0.126V](/tpl/images/0391/6528/96123.png)
![E^0=E^0_{[Pb^{2+}/Pb]}- E^0_{[Zn^{2+}/Zn]}](/tpl/images/0391/6528/a01eb.png)