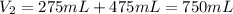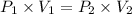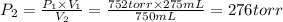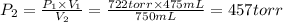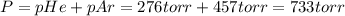A275-ml flask contains pure helium at a pressure of 752 torr. a second flask with a volume of 475 ml contains pure argon at a pressure of 722 torr. if the two flasks are connected through a stopcock and the stopcock is opened, what is the partial pressure of each gas and the total pressure.?

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 10:30
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2

Chemistry, 22.06.2019 14:00
How is the atomic number of a nucleus changed by alpha decay
Answers: 2

Chemistry, 22.06.2019 16:00
If 15 drops of ethanol from a medical dropper weight 0.60g, how many drops does it takes from a dropper to dispense 1.0ml of ethanol? the density of ethanol is 0.80g/ml
Answers: 1
You know the right answer?
A275-ml flask contains pure helium at a pressure of 752 torr. a second flask with a volume of 475 ml...
Questions

Chemistry, 07.07.2019 10:10

Biology, 07.07.2019 10:10

Mathematics, 07.07.2019 10:10


Biology, 07.07.2019 10:10





English, 07.07.2019 10:10


History, 07.07.2019 10:10

Mathematics, 07.07.2019 10:10



English, 07.07.2019 10:10

Mathematics, 07.07.2019 10:10

History, 07.07.2019 10:10