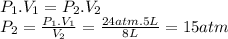Two tanks (tank a and tank b) of gas are connected by a closed valve. tank a is 5 liters and contains o2 gas at a pressure of 24 atm. tank b is 3 liters and contains n2 gas at a pressure of 32 atm. both tanks are held at the same temperature. the valve between the two tanks is opened and the gases are allowed to mix. after the gases have had time to mix, what is the partial pressure of the oxygen gas?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 06:00
Match the name of the following compound: mgso4 · h2omagnesium sulfate monohydratemagnesium (ii) sulfate monohydratemagnesium (ii) sulfate hydratemagnesium sulfate hydrate
Answers: 1

Chemistry, 22.06.2019 15:40
Use the periodic table to complete this equation that represents nuclear fission processesun - ba c 3 n
Answers: 2

Chemistry, 22.06.2019 19:30
To calculate percent by mass, use the equation below: calculate the percent by mass of each element. %n = % %h = % %o = %
Answers: 3

Chemistry, 22.06.2019 21:00
Write a balanced equation showing the formation of copper (ii) nitrite from its elements
Answers: 1
You know the right answer?
Two tanks (tank a and tank b) of gas are connected by a closed valve. tank a is 5 liters and contain...
Questions



Health, 23.02.2021 02:10

Business, 23.02.2021 02:10


Mathematics, 23.02.2021 02:10




Mathematics, 23.02.2021 02:10





Mathematics, 23.02.2021 02:10

Mathematics, 23.02.2021 02:10




Mathematics, 23.02.2021 02:10