Chemistry, 26.11.2019 21:31 jiboyajordan2069
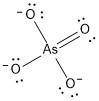
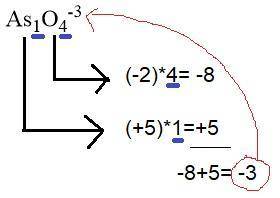
Draw a lewis structure for the resonance form of aso4^-3, with the lowest possible formal charges. include any nonzero formal charges and lone pair electrons in the structure.
-what is the oxidation number of as
-what is the oxidation number of o

Answers: 1
Another question on Chemistry


Chemistry, 21.06.2019 21:30
The density of an unknown gas at 98°c and 740 mmhg is 2.50 g/l. what is the molar mass of the gas with work showed?
Answers: 1

Chemistry, 23.06.2019 02:00
What is the difference between a substance "getting wet" and "being dissolved" in a liquid at the particulate level?
Answers: 3

You know the right answer?
Draw a lewis structure for the resonance form of aso4^-3, with the lowest possible formal charges. i...
Questions

Advanced Placement (AP), 11.11.2020 19:50

Health, 11.11.2020 19:50

Arts, 11.11.2020 19:50


SAT, 11.11.2020 19:50


Chemistry, 11.11.2020 19:50

Physics, 11.11.2020 19:50

Mathematics, 11.11.2020 19:50


Mathematics, 11.11.2020 19:50


Social Studies, 11.11.2020 19:50

English, 11.11.2020 19:50

Mathematics, 11.11.2020 19:50

Mathematics, 11.11.2020 19:50

Spanish, 11.11.2020 19:50

Arts, 11.11.2020 19:50


English, 11.11.2020 19:50