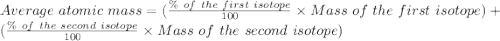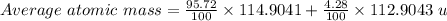Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 00:00
The pressure inside a hydrogen-filled container was 2.10 atm at 21 ? c. what would the pressure be if the container was heated to 92 ? c ?
Answers: 2

Chemistry, 22.06.2019 05:30
Modern weaponry has increased the number of deaths in wars and violent conflicts.
Answers: 3

Chemistry, 23.06.2019 00:00
What does an electron configuration for an atom relate to the atoms placement on the periodic table
Answers: 2

Chemistry, 23.06.2019 01:00
Na chemical reaction, activation energy increases the of the reactants. this outcome causes the particles to collide, which results in the of new products.
Answers: 2
You know the right answer?
An element consists of two isotopes. the abundance of one isotope is 95.72% and its atomic mass is 1...
Questions


Health, 04.02.2020 09:52

Computers and Technology, 04.02.2020 09:52


Spanish, 04.02.2020 09:52

Social Studies, 04.02.2020 09:52



Social Studies, 04.02.2020 09:52

Business, 04.02.2020 09:52







Mathematics, 04.02.2020 09:52

Mathematics, 04.02.2020 09:52

Business, 04.02.2020 09:52

Mathematics, 04.02.2020 09:52