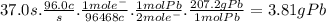Chemistry, 26.11.2019 22:31 maxi12312345
When a lead acid car battery is recharged by the alternator, it acts essentially as an electrolytic cell in which solid lead(ii) sulfate pbso4 is reduced to lead at the cathode and oxidized to solid lead(ii) oxide pbo at the anode. suppose a current of 96.0a is fed into a car battery for 37.0 seconds. calculate the mass of lead deposited on the cathode of the battery. round your answer to 3 significant digits. also, be sure your answer contains a unit symbol.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 23:30
For the following dehydrohalogenation (e2) reaction, draw the zaitsev product(s) resulting from elimination involving c3–c4 (i.e., the carbon atoms depicted with stereobonds). show the product stereochemistry clearly. if there is more than one organic product, both products may be drawn in the same box. ignore elimination involving c3 or c4 and any carbon atom other than c4 or c3.
Answers: 3

Chemistry, 22.06.2019 05:00
1)each group 16 element has how many valence electrons? ( )4 ( )6 ( )8 ( )16 2)how many dots appear in the dot structure for calcium ion, ca2+? ( )zero ( )one ( )two ( )eight 3) which of the following atoms forms a cation to obtain an octet of outer shell electrons? ( )magnesium ( )oxygen ( )fluorine ( )helium 4) an al3+ ion contains 13 protons and 10 electrons. ( )true ( )false 5) valence and non-valence electrons are represented in lewis dot structures. ( )true ( )false
Answers: 3

Chemistry, 22.06.2019 09:10
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 09:20
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1
You know the right answer?
When a lead acid car battery is recharged by the alternator, it acts essentially as an electrolytic...
Questions

Social Studies, 22.06.2019 22:30




History, 22.06.2019 22:30

History, 22.06.2019 22:30







English, 22.06.2019 22:30




Mathematics, 22.06.2019 22:30

Biology, 22.06.2019 22:30