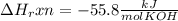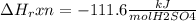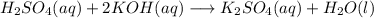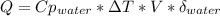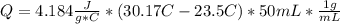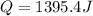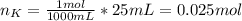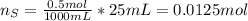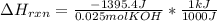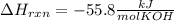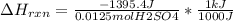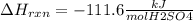When 25.0 ml of 0.500 m h2so4 is added to 25.00 mk of 1.00 m koh in a coffee-cup calorimeter at 23.50◦ c, the temperature rises to 30.17◦ c. calculate dhrxn for this reaction. (assume that the density of water of and specific heat capacity of the solution are the same as for pure water). ( –55.8 kj/mol koh, –112 kj/mol h2so4)

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:00
How much would the freezing point of water decrease if 4 mol of sugar were added to 1 kg of water(k=1.86 c/mol/kg for water and i=1 for sugar
Answers: 1

Chemistry, 22.06.2019 14:30
Which of the following describes a situation where competition between producers exists
Answers: 1

Chemistry, 22.06.2019 14:30
Connect the whole numbers on the periodic table to indicate what they represent?
Answers: 3

Chemistry, 23.06.2019 03:00
What volume does 1.70 ×10–3 mol of chlorine gas occupy if its temperature is 20.2 °c and its pressure is 795 mm hg?
Answers: 3
You know the right answer?
When 25.0 ml of 0.500 m h2so4 is added to 25.00 mk of 1.00 m koh in a coffee-cup calorimeter at 23.5...
Questions

Mathematics, 18.12.2020 06:00


Biology, 18.12.2020 06:00


History, 18.12.2020 06:00


Physics, 18.12.2020 06:00

Physics, 18.12.2020 06:00



Mathematics, 18.12.2020 06:00



History, 18.12.2020 06:00

Chemistry, 18.12.2020 06:00

Advanced Placement (AP), 18.12.2020 06:00




Mathematics, 18.12.2020 06:00