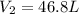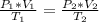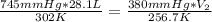Chemistry, 27.11.2019 00:31 happysage12
Aweather balloon is inflated to a volume of 28.1 l at a pressure of 745 mmhg and a temperature of 29.0 ∘c. the balloon rises in the atmosphere to an altitude where the pressure is 380. mmhg and the temperature is -16.3 ∘c. assuming the balloon can freely expand, calculate the volume of the balloon at this altitude.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 21:10
When the volume and number of particles of a gas are constant which of the following is also constant
Answers: 3

Chemistry, 22.06.2019 04:50
Acompound contains c, h, and o atoms. when 1.130 g of the compound is burned in oxygen, 1.064 g co2 and 0.3631 g h2o are produced. what is the empirical formula of this compound?
Answers: 1

Chemistry, 22.06.2019 07:00
The blackbody curve for a star name zeta is shown below. what is the peak wavelength for this star ?
Answers: 1

Chemistry, 22.06.2019 10:30
Which of these is not an example of chemical weathering? a. iron-rich mineral rusting b. feldspar turning into clay c. limestone reacting with acid d. granite breaking up into sand
Answers: 1
You know the right answer?
Aweather balloon is inflated to a volume of 28.1 l at a pressure of 745 mmhg and a temperature of 29...
Questions

Mathematics, 13.11.2020 02:50





Mathematics, 13.11.2020 02:50



Mathematics, 13.11.2020 02:50


English, 13.11.2020 02:50


French, 13.11.2020 02:50


Mathematics, 13.11.2020 02:50

English, 13.11.2020 02:50



History, 13.11.2020 02:50

Mathematics, 13.11.2020 02:50