Chemistry, 27.11.2019 01:31 lattimorekeonna1
If 60.0 grams of carbonic acid are sealed in a 2.00 l soda bottle at room temperature (298 k) and decompose completely via the equation below, what would be the final pressure of carbon dioxide assuming it had the full 2.00 l in which to expand? h₂co₃(aq) → h₂o(l) + co₂(g)

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 21:00
What pressure will be exerted by 0.675 moles of a gas at 25*c if it is in a 0.750-l container?
Answers: 1

Chemistry, 21.06.2019 22:30
Embryos of different species look very similar, which shows that the organisms share a ancestor.
Answers: 1

Chemistry, 22.06.2019 02:30
24 points and brainliest to anyone who can answer under 10 minutes with best ! the table below shows the role of different substances during photosynthesis. substance role during photosynthesis glucose stores chemical energy water combines with glucose to form carbon dioxide chlorophyll traps sunlight which of the following statements would correct one of the roles listed in the table? glucose combines with carbon to form water. chlorophyll reacts with light to produce carbon dioxide. water combines with carbon dioxide during photosynthesis. chlorophyll stores chemical energy needed for photosynthesis.
Answers: 1

Chemistry, 22.06.2019 02:50
Consider the equilibrium system: 2icl(s) ⇄ i2(s) + cl2(g) which of the following changes will increase the total amount of of cl2 that can be produced? all of the listed answers are correct decreasing the volume of the container removing the cl2 as it is formed adding more icl(s) removing some of the i2(s)
Answers: 1
You know the right answer?
If 60.0 grams of carbonic acid are sealed in a 2.00 l soda bottle at room temperature (298 k) and de...
Questions

Social Studies, 05.05.2021 14:00


English, 05.05.2021 14:00



Mathematics, 05.05.2021 14:00

Mathematics, 05.05.2021 14:00

Business, 05.05.2021 14:00


Computers and Technology, 05.05.2021 14:00

Mathematics, 05.05.2021 14:00

English, 05.05.2021 14:00

Mathematics, 05.05.2021 14:00


History, 05.05.2021 14:00

World Languages, 05.05.2021 14:00

English, 05.05.2021 14:00


History, 05.05.2021 14:00

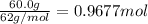
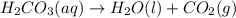
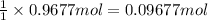 of carbon dioxide
of carbon dioxide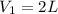
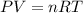 (ideal gas law)
(ideal gas law)