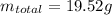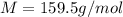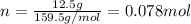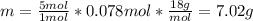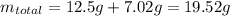Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 12:10
If a molecule with a molecular formula of c13h18 is treated with an excess of h2 in the presence of finally divided pt metal under conditions required for maximum hydrogenation of the molecule to give a molecule with a formula c13h24, how many rings are in the molecule?
Answers: 3

Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be
Answers: 1

Chemistry, 22.06.2019 21:00
The rate constant for the reaction below is 6.2 x 10−5 mol l−1 s −1. if the initial concentration of a is 0.0500 m, what is its concentration after 115 s?
Answers: 1

Chemistry, 22.06.2019 21:00
As we move from left to right across the periodic table, what is the general trend? a) atomic radii increase. b) electronegavitiy decreases. c) nuclear shielding increases. d) metallic character decreases.
Answers: 1
You know the right answer?
If the concentration of a saturated solution at 0∘c is 12.5 gcuso4/100 g soln, what mass of cuso4⋅5h...
Questions

Computers and Technology, 19.07.2019 01:30




Social Studies, 19.07.2019 01:30

English, 19.07.2019 01:30

Arts, 19.07.2019 01:30

Social Studies, 19.07.2019 01:30


History, 19.07.2019 01:30


Social Studies, 19.07.2019 01:30

Biology, 19.07.2019 01:30


Biology, 19.07.2019 01:30

Social Studies, 19.07.2019 01:30


Computers and Technology, 19.07.2019 01:30


Health, 19.07.2019 01:30