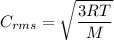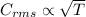Chemistry, 27.11.2019 01:31 dan20012001
If the absolute temperature of a gas is tripled, what happens to the root‑mean‑square speed of the molecules?
a. nothing happens to the rms speed.
b. the new rms speed is 9 times the original rms speed.
c. the new rms speed is 3 times the original rms speed.
d. the new rms speed is 1.732 times the original rms speed.
e. the new rms speed is 1/3 the original rms speed.

Answers: 1
Another question on Chemistry



Chemistry, 22.06.2019 06:40
Which statement correctly describes metallic bonds? a. they form when certain atoms lose electrons and other atoms gain electrons. b. they involve an attraction between anions and cations. they always involvpoth a metal and a nonmetal. d. they can only form between atoms of the same element. e. they form because electrons can move freely between atoms.
Answers: 3

Chemistry, 22.06.2019 14:50
Which of the following is most likely true about water in chemical systems? a) water dissolves nonpolar ionic compounds. b) water dissociates ionic compounds. c) water dissociates covalent molecules. d) water dissolves nonpolar covalent substances.
Answers: 1
You know the right answer?
If the absolute temperature of a gas is tripled, what happens to the root‑mean‑square speed of the m...
Questions

Mathematics, 23.06.2019 23:50



Mathematics, 23.06.2019 23:50

Chemistry, 23.06.2019 23:50













Social Studies, 23.06.2019 23:50