
Chemistry, 27.11.2019 02:31 merrymary3000
The balanced equation for the reaction of bromate ion with bromide in acidic solution is  at a particular instant in time, the rate of disappearance of br– is 2.0 x 10⁻³ mol/l • s. what is the rate of appearance of br₂ at the same instant?
at a particular instant in time, the rate of disappearance of br– is 2.0 x 10⁻³ mol/l • s. what is the rate of appearance of br₂ at the same instant?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 18:40
What kind of ion is contained in salts that produce an acidic solution? a positive ion that attracts a proton from water a positive ion that releases a proton to water a negative ion that attracts a proton from water a negative ion that releases a proton to water
Answers: 1



Chemistry, 23.06.2019 00:20
What type of context clue you understand the meaning of quandary?
Answers: 3
You know the right answer?
The balanced equation for the reaction of bromate ion with bromide in acidic solution is [tex]bro^-...
Questions


Geography, 06.11.2019 18:31

Physics, 06.11.2019 18:31

English, 06.11.2019 18:31






Mathematics, 06.11.2019 18:31



English, 06.11.2019 18:31




Computers and Technology, 06.11.2019 18:31

Mathematics, 06.11.2019 18:31


Social Studies, 06.11.2019 18:31

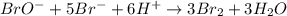
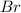 :
: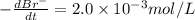
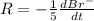
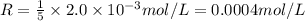
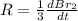
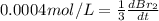
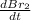 =3\times 0.0004 mol/L=0.0012 mol/L[/tex]
=3\times 0.0004 mol/L=0.0012 mol/L[/tex]

