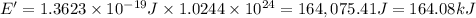Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 02:30
Select each correct answer. more than one answer may be correct. which of the following is a characteristic of unicellular organisms? they can possess tissues and organs. all of their functions are performed by a single cell. they are usually microscopic. each of their cells is specialized to perform a specific function.
Answers: 1

Chemistry, 22.06.2019 18:00
Many pharmaceutical drugs are organic compounds that were originally synthesized in the laboratory. which two scientific disciplines are bridged by pharmaceutical drugs? chemistry and forensics chemistry and medicine biology and forensics biology and criminology
Answers: 2


Chemistry, 23.06.2019 00:00
The empirical formula of a compound is ch2o and its mass is 120 amu/molecule, what is its formula?
Answers: 2
You know the right answer?
Determine the energy of 2.00 mol of photons for each of the following kinds of light. (assume three...
Questions

Biology, 19.07.2019 16:10



Computers and Technology, 19.07.2019 16:10

Physics, 19.07.2019 16:10


Social Studies, 19.07.2019 16:10

History, 19.07.2019 16:10

Social Studies, 19.07.2019 16:10


Chemistry, 19.07.2019 16:10

Social Studies, 19.07.2019 16:10


History, 19.07.2019 16:10


Social Studies, 19.07.2019 16:10

Biology, 19.07.2019 16:10

Mathematics, 19.07.2019 16:10


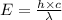
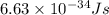
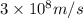
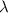 = wavelength =
= wavelength = 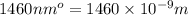
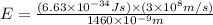
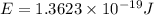
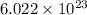 particles
particles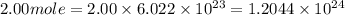 photons
photons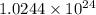 photons = E'
photons = E'