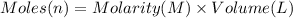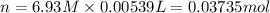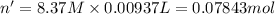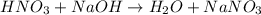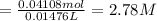Calculate the concentration of acid(or base) remaining in solution when 5.39ml of 6.93m hno3 is added to 9.37ml of 8.37m naoh. note the final volume is the sum of two added volumes. which of the following statement is true for the solution after mixing? a) naoh is in excess overhno3b)hno3 is in excess over naohc)hno3 and naoh are exactly balanced. what is the concentration of the excess naoh (or hno3) you indicated above?

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 16:00
Select the correct answer. you have a nightlight plugged into an outlet in the hallway, which uses 3.5 watts when plugged in. if the house circuit provides 120.0 volts, what is the current through this bulb?
Answers: 1

Chemistry, 21.06.2019 21:30
Aphysical reaction is a process in which one or more reactants change into one or more products with different properties. select the best answer from the choices provided t f
Answers: 1

Chemistry, 22.06.2019 02:20
6. what does the symbol ah stand for? o one calorie given off by a reaction the specific heat of a substance the heat capacity of a substance the heat of reaction for a chemical reaction
Answers: 1

You know the right answer?
Calculate the concentration of acid(or base) remaining in solution when 5.39ml of 6.93m hno3 is adde...
Questions




Biology, 02.10.2019 02:00




History, 02.10.2019 02:00

History, 02.10.2019 02:00


Mathematics, 02.10.2019 02:00

Mathematics, 02.10.2019 02:00

History, 02.10.2019 02:00



Computers and Technology, 02.10.2019 02:00

Mathematics, 02.10.2019 02:00



 .
.