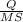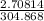If 50.00 ml of 1.05 m sodium hydroxide is added to 25.00 ml of 1.88 m hydrochloric acid, with both solutions originally at 24.66°c, what will be the final solution temperature? (assume that no heat is lost to the surrounding air and that the solution produced in the neutralization reaction has a density of 1.02 g/ml and a specific heat of 3.98 jg⁻¹°c⁻¹.)

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 01:40
C3h8o3 - glycerol major species present when dissolved in water
Answers: 2

Chemistry, 22.06.2019 14:30
An object resting on a table weighs 100 n. with what force is the object pushing on the table? with what force is the table pushing on the object? explain how you got your answer.
Answers: 3

Chemistry, 22.06.2019 17:30
Consider the story you just read. all but one of the choices below indicate that something is living.
Answers: 1

Chemistry, 22.06.2019 18:10
Measurements that have similar values are: a. usually accurate b. sometimes accurate c. always accurate d. never accurate
Answers: 1
You know the right answer?
If 50.00 ml of 1.05 m sodium hydroxide is added to 25.00 ml of 1.88 m hydrochloric acid, with both s...
Questions


History, 21.08.2019 19:30







Mathematics, 21.08.2019 19:30

History, 21.08.2019 19:30






Biology, 21.08.2019 19:30




Mathematics, 21.08.2019 19:30