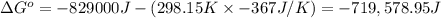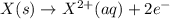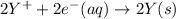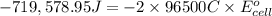Chemistry, 27.11.2019 04:31 rostecorralmart
Calculate the standard cell potential at 25 ∘c for the reactionx(s)+2y+(aq)→x2+(aq)+2y(s)w here δh∘ = -829 kj and δs∘ = -367 j/k

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:00
The graph above shows how the price of cell phones varies with the demand quantity. the equilibrium price for cell phones is where both supply and demand quantities equal $100, 5,000 5,000, $100
Answers: 2

Chemistry, 22.06.2019 05:30
You are making a solution of calcium chloride dissolved in water. you add solid, stir, and it dissolves. you add just a spatula tip full, stir, and the solid does not dissolve. how could you describe the solutions before and after adding the spatula tip amount
Answers: 1

Chemistry, 22.06.2019 13:40
Can someone me with 6 to 10 plz this is for masteries test.
Answers: 1

Chemistry, 22.06.2019 14:30
The three types is stress that act on earths rocks are compression, tension, and
Answers: 1
You know the right answer?
Calculate the standard cell potential at 25 ∘c for the reactionx(s)+2y+(aq)→x2+(aq)+2y(s)w here δh∘...
Questions

Social Studies, 22.07.2019 23:00



















Biology, 22.07.2019 23:00


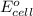 = standard electrode potential of the cell
= standard electrode potential of the cell