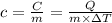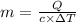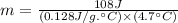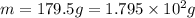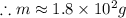Chemistry, 27.11.2019 19:31 jojo171717
If i apply 0.108 kj of energy in order to increase the temperature of a bar of gold from 30.0°c to 34.7°c, and the specific heat capacity of gold is 0.128 j/g°c, what is the mass the bar of gold in grams? a. 1.8 × 102 g b. 6.5 × 101 g c. 1.08 × 102 g d. 1.28 × 102 g e. none of these

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 09:30
Which formula can be used to calculate the molar mass of hydrogen peroxide
Answers: 1

Chemistry, 22.06.2019 11:00
Which statement is true about hcl? (5 points) select one: a. it is a salt because it increases the concentration of metallic ions. b. it is a salt because it is formed by the reaction of an acid and a base. c. it is an acid because it increases the concentration of hydroxyl ions. d. it is an acid because it increases the concentration of hydronium ions.
Answers: 1

Chemistry, 22.06.2019 12:00
What does a complete balanced chemical equation include? a. exothermic coefficients b. endothermic coefficients c. valence electrons d. molar coefficients
Answers: 1

Chemistry, 22.06.2019 12:50
The number at the end of an isotope’s name is the number.
Answers: 1
You know the right answer?
If i apply 0.108 kj of energy in order to increase the temperature of a bar of gold from 30.0°c to 3...
Questions


Spanish, 06.07.2019 01:00









Mathematics, 06.07.2019 01:00





Mathematics, 06.07.2019 01:00



English, 06.07.2019 01:00