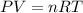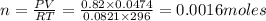Chemistry, 27.11.2019 21:31 robert7248
In an electrolysis experiment such as the one you are going to perform, 47.4 ml of hydrogen gas were collected. the temperature was 23 degrees celsius and the barometric pressure was 643 mm hg. the vapor pressure of water at 23 degrees celsius is 21 mm hg. the number of moles of hydrogen gas formed in the experiment was

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 11:00
The diagram below shows the different phase transitions that occur in matter. which arrow represents the transition in which dew is formed?
Answers: 1

Chemistry, 22.06.2019 11:00
Iron (3) oxide will decompose in the presence of hydrogen gas and heater to produced iron and digydrogen monoxide white a balanced chemical equation
Answers: 1

Chemistry, 22.06.2019 15:30
Which suspect most likely committed the robbery and how do you know
Answers: 2

Chemistry, 22.06.2019 18:00
Mercury turns to vapor at 629.88 k how much heat is lost 175 g of mercury vapor at 650 current condenses to a liquid at 297 ca mercury turns to weber at 629.88 kelvin how much he is lost 175 g of mercury vapor and 650 coming condensers to liquidate 297 kevin
Answers: 2
You know the right answer?
In an electrolysis experiment such as the one you are going to perform, 47.4 ml of hydrogen gas were...
Questions




Chemistry, 10.08.2021 20:10

Mathematics, 10.08.2021 20:10

Mathematics, 10.08.2021 20:10

Chemistry, 10.08.2021 20:10



Biology, 10.08.2021 20:10

English, 10.08.2021 20:10



English, 10.08.2021 20:20

Mathematics, 10.08.2021 20:20


Chemistry, 10.08.2021 20:20

Physics, 10.08.2021 20:20