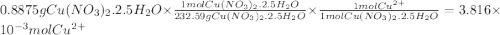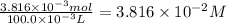Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 08:00
Why is the bond angle in a water molecule less than the bond angle of methane? a. the central oxygen atom in water has two lone pairs of electrons, whereas the central carbon atom in methane has no lone pairs. b. the central hydrogen atom in water has one lone pair of electrons, whereas the central carbon atom in methane has two lone pairs. c. the central oxygen atom in water has four lone pairs of electrons, whereas the central carbon atom in methane has only one lone pair. d. the central oxygen atom exerts more repulsive force on surrounding atoms than the central carbon atom in methane does. reset next
Answers: 2

Chemistry, 22.06.2019 08:40
Write the formula for the following chemicals. 7. e. trinitrogen tetraoxide a calcium phosphate f. magnesium acetate b. potassium sulfide g nickel(iii) cyanide c carbon dioxide h. silver sulfate d. cobalt(ii) chloride
Answers: 1

Chemistry, 22.06.2019 13:30
Mary is conducting an experiment on how pollution affects plant growth. how can she ensure that her data are reliable?
Answers: 3

Chemistry, 22.06.2019 20:10
What would happen to a volleyball left outside in the winter? o o o o a. it would expand. b. it would lose air. c. it would shrink. d. it would explode.
Answers: 2
You know the right answer?
Astock solution of cu2+(aq) was prepared by placing 0.8875 g of solid cu(no3)2∙2.5 h2o in a 100.0-ml...
Questions

Spanish, 01.08.2019 23:00


Spanish, 01.08.2019 23:00




Mathematics, 01.08.2019 23:00


History, 01.08.2019 23:00


Advanced Placement (AP), 01.08.2019 23:00


Biology, 01.08.2019 23:00



Physics, 01.08.2019 23:00

Chemistry, 01.08.2019 23:00

Health, 01.08.2019 23:00