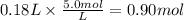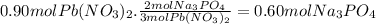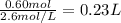Chemistry, 27.11.2019 22:31 marelinatalia2000
Assume 0.18 l of a 5.0 m solution of lead (ii) nitrate, pb(no3)2, reacts with a 2.6 m solution of sodium phosphate, na3po4, to produce lead (ii) phosphate, pb3(po4)2, and sodium nitrate, nano3. the problem requires that you determine the volume of sodium phosphate, na3po4, needed for the reaction to occur.

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 02:40
How many liters of hydrogen gas will be produced at stp from the reaction of 7.179×10^23 atoms of magnesium with 54.219g of phosphoric acid (h3po4) the equation is 3mg + 2h3(> mg(po4)2+3h2
Answers: 1


Chemistry, 22.06.2019 19:20
15. which of the following is not human-caused groundwater pollution? a. water in an aquifer dissolves elements such as arsenic and mercury from surrounding rock. b. water in an aquifer is contaminated by leachate that seeps into the ground from a landfill. c. water in an aquifer becomes polluted with chemicals used in hydraulic fracturing, or fracking. d. water in an aquifer absorbs harmful bacteria from the drainage field of a septic tank.
Answers: 1
You know the right answer?
Assume 0.18 l of a 5.0 m solution of lead (ii) nitrate, pb(no3)2, reacts with a 2.6 m solution of so...
Questions


Social Studies, 10.04.2021 02:20



Mathematics, 10.04.2021 02:20

Mathematics, 10.04.2021 02:20

Mathematics, 10.04.2021 02:20

English, 10.04.2021 02:20

History, 10.04.2021 02:20

Biology, 10.04.2021 02:20

Mathematics, 10.04.2021 02:20


History, 10.04.2021 02:20



Computers and Technology, 10.04.2021 02:20


Social Studies, 10.04.2021 02:20


English, 10.04.2021 02:20