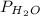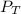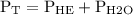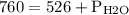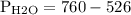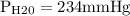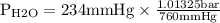Chemistry, 27.11.2019 22:31 Roberto2014
Asealed glass jar has a small amount of water on the bottom. the rest of the volume in the jar is taken up by helium gas at a partial pressure of 526 mmhg, and water vapor. if the total pressure inside the jar is 760. mmhg, what is the temperature of the water?

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 22:00
Describe the particles of all three phases of matter in the root beer float. (how are the particles arranged and moving? )
Answers: 3

Chemistry, 21.06.2019 23:00
At room temperature what happens to the average kinetic energy of the molecules of a solid, liquid, and a gas
Answers: 2

Chemistry, 22.06.2019 05:00
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change
Answers: 3

Chemistry, 22.06.2019 12:30
Place the elements below in order of decreasing ionization energy. aluminum(al) chlorine(cl) magnesium (mg) sulfur(s)
Answers: 1
You know the right answer?
Asealed glass jar has a small amount of water on the bottom. the rest of the volume in the jar is ta...
Questions






Mathematics, 16.10.2019 13:30




Mathematics, 16.10.2019 13:30


History, 16.10.2019 13:30





Business, 16.10.2019 13:30

Mathematics, 16.10.2019 13:30


Social Studies, 16.10.2019 13:30

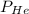 and partial pressure of water is
and partial pressure of water is