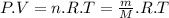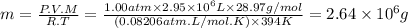Chemistry, 27.11.2019 23:31 antionette1
Ahot air balloon can have a volume of 2950 cubic meters (2.95 ✕ 106 liters) and operate at temperatures up to 250°f (121°c). assuming a balloon is operating at this maximum temperature with an external pressure of 1.00 atm, what mass of air would the balloon hold? while air is a mixture of many gases, we can assume a molar mass of 28.97 g/mol for air.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 02:10
When 225mg of anthracene, c14h10(s), was burned in a bomb calorimeter the temperature rose by 1.75k. calculate the calorimeter constant. by how much will the temperature rise when 125mg of phenol, c6h5oh(s), is burned in the calorimeter under the same conditions? (δch< (c14h10,s)=–7061 kj mol−1.)
Answers: 3

Chemistry, 22.06.2019 08:30
Analyze how limestone is weathered and identify the features that are formed as a result of this dissolution
Answers: 1

Chemistry, 23.06.2019 00:20
What type of context clue you understand the meaning of quandary?
Answers: 3

Chemistry, 23.06.2019 07:30
How many moles of aluminum chloride are produced from 2.5 moles of magnesium chloride
Answers: 1
You know the right answer?
Ahot air balloon can have a volume of 2950 cubic meters (2.95 ✕ 106 liters) and operate at temperatu...
Questions


English, 22.11.2019 11:31

English, 22.11.2019 11:31

Mathematics, 22.11.2019 11:31

English, 22.11.2019 11:31

Mathematics, 22.11.2019 11:31


Mathematics, 22.11.2019 11:31

Mathematics, 22.11.2019 11:31

History, 22.11.2019 11:31


Social Studies, 22.11.2019 11:31

English, 22.11.2019 11:31

English, 22.11.2019 11:31

Mathematics, 22.11.2019 11:31




Mathematics, 22.11.2019 11:31

Mathematics, 22.11.2019 11:31