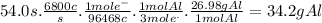Chemistry, 28.11.2019 00:31 angeljaylyn123
In the hall-heroult process, a large electric current is passed through a solution of aluminum oxide (a12o 3) dissolved in molten cryolite (na3aif6) ,resulting in the reduction of the ai�3 to pure aluminum. suppose a current of 6800.a is passed through a hall-heroult cell for 54.0 seconds. calculate the mass of pure aluminum produced. be sure your answer has a unit symbol and the correct number of significant digits.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 07:30
Compare and contrast the bohr model and the electron cloud models of the atom.
Answers: 1

Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3

Chemistry, 22.06.2019 16:20
When water dissolves sugar, which process is not involved? o dissociation o hydration o surface area of the solute increases sa
Answers: 1

Chemistry, 22.06.2019 19:00
How does kepler second law of planetary motion overthrow one of the basic beliefs of classical astronomy
Answers: 1
You know the right answer?
In the hall-heroult process, a large electric current is passed through a solution of aluminum oxide...
Questions

Business, 26.12.2019 01:31

Social Studies, 26.12.2019 01:31



Social Studies, 26.12.2019 01:31




Mathematics, 26.12.2019 01:31







Chemistry, 26.12.2019 01:31

Mathematics, 26.12.2019 01:31

Mathematics, 26.12.2019 01:31

Social Studies, 26.12.2019 01:31