
Chemistry, 28.11.2019 04:31 dbn4everloved8
A1.00 g sample of n-hexane (c6h14) undergoes complete combustion with excess o2 in a bomb calorimeter. the temperature of the 1502 g of water surrounding the bomb rises from 22.64°c to 29.30°c. the heat capacity of the hardware component of the calorimeter (everything that is not water) is 4042 j/°c. what is δu for the combustion of n-c6h14? one mole of n-c6h14 is 86.1 g. the specific heat of water is 4.184 j/g·°c.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 17:30
How many grams of magnesium metal will react completely with 8.3 liters of 5.5 m hcl? show all of the work needed to solve this problem. mg (s) + 2hcl (aq) → mgcl2 (aq) + h2 (g)
Answers: 3

Chemistry, 22.06.2019 00:00
Draw the skeletal structures of two different molecules that are each made of 5 carbon atoms and 12 hydrogen atoms.
Answers: 1


Chemistry, 22.06.2019 14:00
What term describes technology that operates on an atomic level
Answers: 2
You know the right answer?
A1.00 g sample of n-hexane (c6h14) undergoes complete combustion with excess o2 in a bomb calorimete...
Questions

Business, 01.07.2019 09:00


Chemistry, 01.07.2019 09:00



History, 01.07.2019 09:00

Mathematics, 01.07.2019 09:00

Chemistry, 01.07.2019 09:00



Physics, 01.07.2019 09:00

English, 01.07.2019 09:00

History, 01.07.2019 09:00




English, 01.07.2019 09:00



History, 01.07.2019 09:00

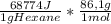 = -5,921x10⁶J/mol
= -5,921x10⁶J/mol

