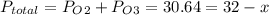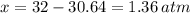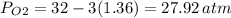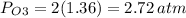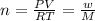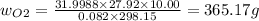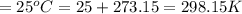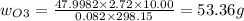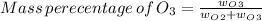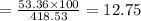Chemistry, 28.11.2019 04:31 brandon2222269
An evacuated steel vessel with a volume of 10.00 liters is filled with 32.00 atm ofpure o2 at 25°c. an electric discharge is passed through the vessel, causing someof the oxygen to be converted into ozone. as a result, the pressure inside thevessel drops to 30.64 atm at 25°c. calculate the final percent by mass of ozonein the vessel

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 06:00
In an investigation that uses the scientific method, which step immediately follows making a hypothesis? o summarizing the results o asking a question o making observations designing an experiment mark this and retum save and exit next submit
Answers: 2

Chemistry, 22.06.2019 13:50
Amap that uses a range of colors and shading to represent the elevation, depth, or landscape of specific features on earth is a/an map.
Answers: 3

Chemistry, 22.06.2019 22:00
8) warming your hands by a fire is an example if which heat transfer? a. conduction b. convection c. radiation d. none of these
Answers: 1

Chemistry, 23.06.2019 03:30
How many grams of sodium chloride are in 250ml of a 2.5m naci solution
Answers: 1
You know the right answer?
An evacuated steel vessel with a volume of 10.00 liters is filled with 32.00 atm ofpure o2 at 25°c....
Questions



Mathematics, 05.10.2020 01:01




Physics, 05.10.2020 01:01

History, 05.10.2020 01:01

Mathematics, 05.10.2020 01:01

History, 05.10.2020 01:01


History, 05.10.2020 01:01



Mathematics, 05.10.2020 01:01


Mathematics, 05.10.2020 01:01



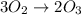
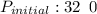 (Here, molar mass of oxygen molecule = 32 g/mol)
(Here, molar mass of oxygen molecule = 32 g/mol)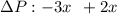
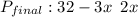
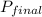 = 30.64
= 30.64