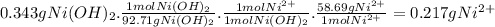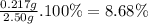To measure the amount of nickel in some industrial waste fluid, an analytical chemist adds 0.110 m sodium hydroxide (naoh) solution to a 25.0 g sample of the fluid and collects the solid nickel(i) hydroxide (ni (oh2) product. when no more ni(oh)2 is produced, he filters, washes and weighs it, and finds that 343. mg has been produced the balanced chemical equation for the reaction is: ni2+(aq) + 2naoh(aq) ni(oh)2(s) + 2 na. (ag) r precipitation | | o acid-base o redox og dx10 ar what kind of reaction is this? if you said this was a precipitation reaction, enter the chemical formula of the precipitate. if you said this was an acid-base reaction, enter the chemical formula of the reactant that is acting as the base. if you said this was a redox reaction, enter the chemical symbol of the element that is oxidized. calculate the mass percent of ni in the sample. be sure your answer has the correct number of significant digits.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:40
How many electrons does silver have to give up in order to achieve a sido noble gas electron configuration?
Answers: 3

Chemistry, 22.06.2019 14:00
Calculate the energy required to ionize a hydrogen atom to an excited state where the electron is initially in the n = 5 energy level. report your answer in kilojoules
Answers: 1

Chemistry, 22.06.2019 16:30
An atom with 7 protons, 6 neutrons, and 7 electrons has an atomic mass of amu. (enter a whole number.) numerical answers expected! answer for blank 1:
Answers: 3

Chemistry, 22.06.2019 22:00
11) burning your hand when accidentally touching a hot plate is an example of which heat transfer? a. conduction b. convection c. radiation d. none of these
Answers: 2
You know the right answer?
To measure the amount of nickel in some industrial waste fluid, an analytical chemist adds 0.110 m s...
Questions

History, 10.12.2020 23:00


Mathematics, 10.12.2020 23:00

Arts, 10.12.2020 23:00


Biology, 10.12.2020 23:00

Mathematics, 10.12.2020 23:00

Biology, 10.12.2020 23:00

Geography, 10.12.2020 23:00



SAT, 10.12.2020 23:00

Mathematics, 10.12.2020 23:00

Mathematics, 10.12.2020 23:00


Spanish, 10.12.2020 23:00

English, 10.12.2020 23:00


Engineering, 10.12.2020 23:00

English, 10.12.2020 23:00