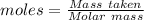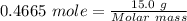Chemistry, 28.11.2019 05:31 yurionice42069
Silane is a gas that is used to prepare extremely pure silicon for use in semiconductors, such as computer chips. a sample of silane has a mass of 15.0 g and occupies a volume of 4.81 l at a pressure of 2.35 atm and a temperature of 22 ºc. what is the molar mass of silane?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:00
If the particles in a sample of matter have an orderly arrangement and move only in place, the sample is a
Answers: 1

Chemistry, 22.06.2019 10:30
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1

Chemistry, 22.06.2019 22:10
Determine the ph of 0.10 m nh3 solution. nh3 is a weak base with a kb equal to 1.8 x 10-5 round answer to nearest whole number.
Answers: 1

Chemistry, 22.06.2019 23:30
The density of benzene at 15 °c is 0.8787 g/ml. calculate the mass of 0.1500 l of benzene at this temperature. enter your answer in terms of grams
Answers: 2
You know the right answer?
Silane is a gas that is used to prepare extremely pure silicon for use in semiconductors, such as co...
Questions



English, 07.12.2020 03:40


Business, 07.12.2020 03:40


Mathematics, 07.12.2020 03:40



Biology, 07.12.2020 03:40







English, 07.12.2020 03:40


Mathematics, 07.12.2020 03:40

Biology, 07.12.2020 03:40