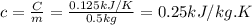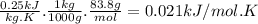Chemistry, 28.11.2019 05:31 emmazhu1106
When 18.9 kj is transferred to a gas sample in a constant volume adiabatic container with a calorimeter constant of 2.22 kj/k, the temperature of the gas (and the calorimeter) increases by 8.06 k. (a) what is the heat capacity of the sample? (b) if the sample has a mass of 0.5 kilograms, what is the specific heat capacity of the substance? (c) if the sample is krypton, what is the molar heat capacity at constant volume of krypton? the molar mass of krypton is 83.8 grams/mole.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 14:50
Your roll: experienced electron speech is adressed to: a new "freshman class" of electrons job: write a speech task: you are to pretend that you are giving a speech to a new group of electrons. be sure to mention their placement in an atom, their charge, and their role in chemical bonding (ionic and covalent) be specific!
Answers: 3

Chemistry, 22.06.2019 02:50
Consider the equilibrium system: 2icl(s) ⇄ i2(s) + cl2(g) which of the following changes will increase the total amount of of cl2 that can be produced? all of the listed answers are correct decreasing the volume of the container removing the cl2 as it is formed adding more icl(s) removing some of the i2(s)
Answers: 1

Chemistry, 22.06.2019 17:00
What is the approximate vapor pressure when the gas condenses at 70 degrees celsius
Answers: 2

Chemistry, 22.06.2019 22:30
What is the work done by the electric force to move a 1 c charge from a to b?
Answers: 2
You know the right answer?
When 18.9 kj is transferred to a gas sample in a constant volume adiabatic container with a calorime...
Questions

English, 22.06.2021 17:30





Mathematics, 22.06.2021 17:30



Biology, 22.06.2021 17:30


History, 22.06.2021 17:30

Mathematics, 22.06.2021 17:30

History, 22.06.2021 17:30

English, 22.06.2021 17:30

Mathematics, 22.06.2021 17:30



Health, 22.06.2021 17:30


Biology, 22.06.2021 17:30