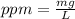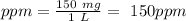Chemistry, 28.11.2019 05:31 jellyangie1
Astock solution contains a mixture of ~100 ppm chloride, fluoride, nitrite, bromide, nitrate and phosphate anions. in order to prepare 1 l of 100 ppm nitrite stock solution, you weigh out 150.0 mg of nano2. the actual concentration of nitrite would be:

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 23:50
How does a scientist the government? a. the scientist tells people in society what to do. b. the scientist determines the policies that the government spends money on. c. the scientist provides unbiased information to the government. d. the scientist makes laws based on his or her research results.
Answers: 1

Chemistry, 22.06.2019 07:30
Given that 1 mi = 1760 yd, determine what conver- sion factor is appropriate to convert 1849 yd to miles; to convert 2.781 mi to yards.
Answers: 2

Chemistry, 22.06.2019 09:00
What is the percentage composition of carbon in the compound ch4
Answers: 1

You know the right answer?
Astock solution contains a mixture of ~100 ppm chloride, fluoride, nitrite, bromide, nitrate and pho...
Questions




Mathematics, 24.06.2019 02:00






History, 24.06.2019 02:00



Mathematics, 24.06.2019 02:00


Social Studies, 24.06.2019 02:00


History, 24.06.2019 02:00



Geography, 24.06.2019 02:00

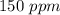
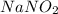 , so:
, so: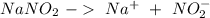
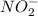 is 1:1
is 1:1