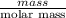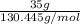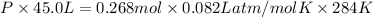Chemistry, 28.11.2019 05:31 cookies1164
Areaction between liquid reactants takes place at - 11.0 degree c in a sealed, evacuated vessel with a measured volume of 45.0 l. measurements show that the reaction produced 35. g of chlorine pentafluoride gas. calculate the pressure of chlorine pentafluoride gas in the reaction vessel after the reaction. you may ignore the volume of the liquid of reactants. be sure your answer has the correct number of significant digits.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 17:50
Cryolite, na3alf6(s), an ore used in the production of aluminum, can be synthesized using aluminum oxide. start this question by first balance the chemical equation.1.) balance the equation: - alo3(s)+naoh(l)+hf(> na3alf6+h2o(g). 2.) if 17.5 kilograms of al2o3(s), 51.4 kilograms of naoh(l), and 51.4 kilograms of hf(g) react completely, how many kilograms of cryolite will be produced? 3.)which reactants will be in excess, (al2o3, naoh, or hf) 4.)what is the total mass of the excess reactants left over after the reaction is complete in kg?
Answers: 2

Chemistry, 22.06.2019 21:20
The organs inside the body and how they function together
Answers: 3

Chemistry, 23.06.2019 04:10
Which of the following is described by the equation h2o(s)+ heat=h2o(i) a freezing melting condensing evaporating
Answers: 2

Chemistry, 23.06.2019 06:00
What physical property of gold makes panning a useful way to get gold from streams?
Answers: 2
You know the right answer?
Areaction between liquid reactants takes place at - 11.0 degree c in a sealed, evacuated vessel with...
Questions

Health, 06.10.2021 01:20

Mathematics, 06.10.2021 01:20

Mathematics, 06.10.2021 01:20

Mathematics, 06.10.2021 01:20

English, 06.10.2021 01:20

Mathematics, 06.10.2021 01:20

Mathematics, 06.10.2021 01:20



Mathematics, 06.10.2021 01:20

Biology, 06.10.2021 01:30


Social Studies, 06.10.2021 01:30

Mathematics, 06.10.2021 01:30



History, 06.10.2021 01:30

Mathematics, 06.10.2021 01:30

History, 06.10.2021 01:30

Biology, 06.10.2021 01:30

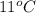 = (11 + 273) K = 284 K, V = 45.0 L
= (11 + 273) K = 284 K, V = 45.0 L