

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 07:00
This image is an example of a(n) a) atom. b) compound. c) mixture. d) molecule.
Answers: 1

Chemistry, 22.06.2019 11:50
Which of the following statements about hybrid orbitals is or are true? choose all that apply. choose all that apply. under sp2 hybridization, the large lobes point to the vertices of an equilateral triangle. after an atom undergoes sp hybridization there is one unhybridized p orbital on the atom. the angle between the large lobes of sp3 hybrids is 109.5∘
Answers: 2

Chemistry, 22.06.2019 19:30
Use the periodic table to find the molar mass of each element. molar mass h = g/mol molar mass s = g/mol molar mass o = g/mol
Answers: 3

You know the right answer?
Which of the following liquids has the greatest viscocity? a. hexane (c6h14) b. ch3och2ch3 c. ch3ch...
Questions




Chemistry, 11.05.2021 19:00

Mathematics, 11.05.2021 19:00





English, 11.05.2021 19:10



Health, 11.05.2021 19:10

Mathematics, 11.05.2021 19:10

Advanced Placement (AP), 11.05.2021 19:10



Chemistry, 11.05.2021 19:10


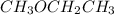 is 60.09 g/molMass of
is 60.09 g/molMass of 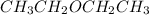 is 74.12 g/molMass of
is 74.12 g/molMass of 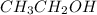 is 40.06 g/mol
is 40.06 g/mol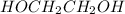 is 62.07 g/mol
is 62.07 g/mol


