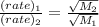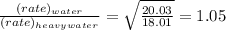Chemistry, 28.11.2019 06:31 99keevintaylor012
84. heavy water, d2o (molar mass = 20.03 g mol–1), can be separated from ordinary water, h2o (molar mass = 18.01), as a result of the difference in the relative rates of diffusion of the molecules in the gas phase. calculate the relative rates of diffusion of h2o and d2o.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 05:50
Why doesn't heat added to water make the tempature rise above 100c
Answers: 2

Chemistry, 22.06.2019 12:00
From the options provided for each element below, choose the properties that it may have based on its location in the periodic table fluorine (f): highly reactive nonmetal shiny a conductor
Answers: 1

Chemistry, 22.06.2019 16:10
Predict the reactants of this chemical reaction. that is, fill in the left side of the chemical equation. be sure the equation you submit is balanced. (you can edit both sides of the equation to balance it, if you need to.) note: you are writing the molecular, and not the net ionic equation. > cacl2(aq) + h20(l)
Answers: 2

Chemistry, 23.06.2019 01:00
Which description best characterization the motion of particles in a solid
Answers: 1
You know the right answer?
84. heavy water, d2o (molar mass = 20.03 g mol–1), can be separated from ordinary water, h2o (molar...
Questions

Chemistry, 26.03.2021 21:30

Mathematics, 26.03.2021 21:30



Biology, 26.03.2021 21:30


Mathematics, 26.03.2021 21:30




Mathematics, 26.03.2021 21:30



English, 26.03.2021 21:30



Biology, 26.03.2021 21:30

Chemistry, 26.03.2021 21:30

Business, 26.03.2021 21:30