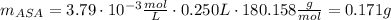Chemistry, 28.11.2019 07:31 oneicyahdaley10
(1.) using beer's law, how will the absorbance measured for the solutions change as the concentration of aspirin in solutions increase?
(2.) in an experiment, the beer’s law plot resulted in the following relationship between absorbance and concentration of asa, y = 1061.5x, where y is absorbance and x is the concentration. if the absorbance of a sample solution prepared from an aspirin tablet is 0.402, calculate the concentration of asa in the solution in m.
(3.) if the above solution was prepared by taking 10 ml of stock solution and diluting it to 100 ml, what is the concentration of the stock solution?
(4.) this stock solution was prepared as follows: an aspirin tablet was transferred to an erlenmeyer flask and reacted with naoh. the resulting solution was transferred to a 250 ml volumetric flask and the volume made up to 250 ml. calculate the mass of aspirin in the tablet based on the concentration of aspirin in the stock solution.

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 20:30
A40 kilogram skier starts at the top of a 12 meter high slope. at the bottom, she is travelling 10 meters per second. how much energy does she lose to friction
Answers: 2

Chemistry, 22.06.2019 23:00
What extra step distinguishes fermentation from glycolysis
Answers: 1

Chemistry, 22.06.2019 23:10
Match the formula for the following compound: magnesium sulfate heptahydratemgs·7h2omg2so4·7h2omg(so4)2·7h2omgso4·7h2o
Answers: 1
You know the right answer?
(1.) using beer's law, how will the absorbance measured for the solutions change as the concentratio...
Questions


History, 21.09.2019 23:30



Mathematics, 21.09.2019 23:30



Mathematics, 21.09.2019 23:30

English, 21.09.2019 23:30


Mathematics, 21.09.2019 23:30

Mathematics, 21.09.2019 23:30


History, 21.09.2019 23:30


Computers and Technology, 21.09.2019 23:30

Mathematics, 21.09.2019 23:30

Biology, 21.09.2019 23:30


English, 21.09.2019 23:30

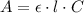 (1)
(1)![A = 1061.5 \cdot [ASA]](/tpl/images/0394/5398/223a2.png)
![[ASA] = \frac{A}{1061.5} = 3.79 \cdot 10^{-4}M](/tpl/images/0394/5398/3c100.png)
![V_{i} [ASA]_{i} = V_{f} [ASA]_{f}](/tpl/images/0394/5398/57531.png)
![[ASA]_{i} = \frac{V_{f} \cdot [ASA]_{f}}{V_{i}} = \frac {100mL \cdot 3.79\cdot 10^{-4} M}{10mL} = 3.79 \cdot 10^{-3} M](/tpl/images/0394/5398/a10be.png)
![m_{ASA} = \eta_{ASA} \cdot M_{ASA} = [ASA]_{i} \cdot V_{0} \cdot M_{ASA}](/tpl/images/0394/5398/9bfc0.png)