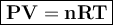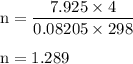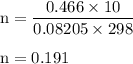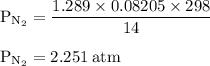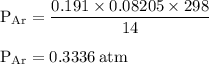Chemistry, 28.11.2019 08:31 lakeshia8880
15. an apparatus consists of a 4.0 dm3
flask containing nitrogen gas at 25 oc and 803 kpa. it is joined
by a valve to a 10.0 dm3
flask containing argon gas 25 oc and 47.2 kpa. the valve is opened and
the gases mix.
i. what is the partial pressure of each gas after mixing?
ii. what is the total pressure of the gas mixture

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 20:30
Which of the following true? a_volcanoes and earthquakes often near the plate boundaries. b_volcanoes occur whereve there are tall mountains. c_earthquakes cause volcanoes in the same location to erupt violently d_volcanoes and earthquakes occur only where plates are colliding with each other
Answers: 2

Chemistry, 22.06.2019 10:00
In a water molecule, hydrogen and oxygen are held together by a(an) bond. a) double covalent b) ionic c) nonpolar covalent d) hydrogen e) polar covalent
Answers: 1

Chemistry, 22.06.2019 13:30
If the concentration of phosphate in the cytosol is 2.0 mm and the concentration of phosphate in the surrounding fluid is 0.1 mm, how could the cell increase the concentration of phosphate in the cytosol? a) passive transportb) diffusionc) active transportd) osmosise) facilitated diffusion
Answers: 3

Chemistry, 22.06.2019 14:30
For the reaction shown, find the limiting reactant for each of the following initial amounts of reactants. 4al(s)+3o2(g)→2al2o3(s) a) 1 molal, 1 mol o2 b) 4 molal, 2.6 mol o2 c) 16 molal, 13 mol o2 d) 7.4 molal, 6.5 mol o2
Answers: 3
You know the right answer?
15. an apparatus consists of a 4.0 dm3
flask containing nitrogen gas at 25 oc and 803 kpa. it...
flask containing nitrogen gas at 25 oc and 803 kpa. it...
Questions


Mathematics, 04.02.2022 23:10


English, 04.02.2022 23:10

Mathematics, 04.02.2022 23:10

Biology, 04.02.2022 23:10


Social Studies, 04.02.2022 23:10

Mathematics, 04.02.2022 23:10



Mathematics, 04.02.2022 23:10

Chemistry, 04.02.2022 23:10

Mathematics, 04.02.2022 23:20

Mathematics, 04.02.2022 23:20



Biology, 04.02.2022 23:20

History, 04.02.2022 23:20

Mathematics, 04.02.2022 23:20