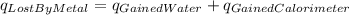In the laboratory a "coffee cup" calorimeter, or constant pressure calorimeter, is frequently used to determine the specific heat of a solid, or to measure the energy of a solution phase reaction. a student heats 61.68 grams of gold to 99.01 °c and then drops it into a cup containing 79.34 grams of water at 22.14 °c. she measures the final temperature to be 23.98 °c. the heat capacity of the calorimeter (sometimes referred to as the calorimeter constant) was determined in a separate experiment to be 1.80 j/°c. assuming that no heat is lost to the surroundings calculate the specific heat of gold.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 06:30
The following reaction shows sodium carbonate reacting with calcium hydroxide. na2co3 + ca(oh)2 → naoh + caco3 how many grams of naoh are produced from 20.0 grams of na2co3? (molar mass of na = 22.989 g/mol, c = 12.01 g/mol, o = 15.999 g/mol, ca = 40.078 g/mol, h = 1.008 g/mol) 12.2 grams 15.1 grams 24.4 grams 30.2 grams
Answers: 2

Chemistry, 22.06.2019 11:00
When hydrochloric acid reacts with potassium hydroxide solution, the following reaction occurs. hcl (aq) + koh (aq) h2o (l) + kcl (aq) the reaction gives off heat energy, so it is an reaction.
Answers: 1

Chemistry, 22.06.2019 11:00
Surface currents are caused by blank space . question 14 options: surface currents are caused by? differences in water temperature high salinity differences in density wind forces
Answers: 1

Chemistry, 22.06.2019 11:40
Which type of precipitation would most likely form when the surface air temperature is slightly below freezing and the air temperature increases as you move upward away from the ground?
Answers: 2
You know the right answer?
In the laboratory a "coffee cup" calorimeter, or constant pressure calorimeter, is frequently used t...
Questions

Mathematics, 14.12.2021 03:00

Physics, 14.12.2021 03:00

SAT, 14.12.2021 03:00

SAT, 14.12.2021 03:00

SAT, 14.12.2021 03:00



Social Studies, 14.12.2021 03:00

Mathematics, 14.12.2021 03:00

Computers and Technology, 14.12.2021 03:00



SAT, 14.12.2021 03:00


Social Studies, 14.12.2021 03:00


SAT, 14.12.2021 03:00

Mathematics, 14.12.2021 03:00