
Chemistry, 28.11.2019 22:31 leannadoughty06
A3.452 g sample containing an unknown amount of a ce(iv) salt is dissolved in 250.0-ml of 1 m h2so4. a 25.00 ml aliquot is analyzed by adding ki and titrating the i3– that forms with s2o32–. the end point was reached following the addition of 13.02 ml of 0.03428 m na2s2o3. calculate the weight percent of ce4 in the sample. when should the indicator be added to this titration? (at the beginning or just before the end point? )

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 15:00
Which are forms of frozen water? check all that apply. dew frost hail rain sleet
Answers: 2

Chemistry, 22.06.2019 15:00
Answer explain why it is not possible to deduce a complete order of reactivity.
Answers: 3

Chemistry, 22.06.2019 18:00
To apply in a gold the individual gold atoms are united to each other by means of a metallic bond. how would you use the gold block to determine the atomic radius of a gold atom?
Answers: 3

Chemistry, 22.06.2019 20:10
Insoluble sulfide compounds are generally black in color. which of the following combinations could yield a black precipitate? check all that apply. na2s(aq)+kcl(aq) li2s(aq)+pb(no3)2(aq) pb(clo3)2(aq)+nano3(aq) agno3(aq)+kcl(aq) k2s(aq)+sn(no3)4(aq)
Answers: 1
You know the right answer?
A3.452 g sample containing an unknown amount of a ce(iv) salt is dissolved in 250.0-ml of 1 m h2so4....
Questions

Mathematics, 03.07.2019 09:40

English, 03.07.2019 09:40




English, 03.07.2019 09:40



Mathematics, 03.07.2019 09:40


Mathematics, 03.07.2019 09:40

Mathematics, 03.07.2019 09:40

English, 03.07.2019 09:40


English, 03.07.2019 09:40

Chemistry, 03.07.2019 09:40

Mathematics, 03.07.2019 09:40

English, 03.07.2019 09:40

Mathematics, 03.07.2019 09:40

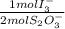 = 2,2315x10⁻⁴ moles of I₃⁻
= 2,2315x10⁻⁴ moles of I₃⁻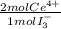 = 4,463x10⁻⁴ moles of Ce(IV). These moles are:
= 4,463x10⁻⁴ moles of Ce(IV). These moles are: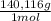 = 0,0625 g of Ce(IV)
= 0,0625 g of Ce(IV)

