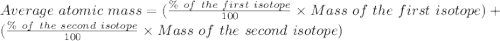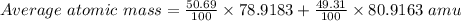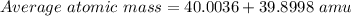Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 12:40
In the following table, all the columns for the element calcium are filled out correctly. element electron structure of atom electron structure of ion net ionic charge calcium 1s22s22p63s23p64s2 1s32s22p63s23p64s1 +1 true false
Answers: 2

Chemistry, 22.06.2019 14:00
Anthracite is so hard and pure it is also referred to as a renewable resource metamorphic rock hot bituminous coal dirty fuel
Answers: 1

Chemistry, 22.06.2019 18:00
Heat is the total potential energy of a substance that can be transferred. true false
Answers: 1

Chemistry, 22.06.2019 19:00
Convert the temperature of dry ice, –77 ∞c, into degrees fahrenheit and kelvin.
Answers: 2
You know the right answer?
Bromine has two isotopes 79br and 81br, whose masses (78.9183 and 80.9163 amu) and abundances (50.69...
Questions



Mathematics, 08.11.2020 01:00


Social Studies, 08.11.2020 01:00



Health, 08.11.2020 01:00

Mathematics, 08.11.2020 01:00

English, 08.11.2020 01:00



Physics, 08.11.2020 01:00


Mathematics, 08.11.2020 01:00

History, 08.11.2020 01:00